Biosciences, Biotechnology
IMBB researchers reveal that DNA damage in tissue-infiltrating macrophages triggers an exosome-based metabolic reprogramming leading to chronic inflammation.
Research carried out at the Institute of Molecular Biology and Biotechnology (IMBB) of FORTH, provides evidence that persistent DNA damage triggers an exosome-based, metabolic reprogramming that leads to chronic inflammation and tissue pathology in DNA repair-deficient progeroid syndromes and likely also during aging.
Inborn defects in DNA repair mechanisms are associated with cancer, aging but also with complex metabolic and endocrine disorders whose causal mechanisms are not well understood. Using animals with a DNA repair, the IMBB researchers provide a novel mechanism by which DNA damage leads to cellular senescence, fibrosis, loss of tissue architecture and chronic pancreatitis in mice. These findings led the team to propose a new therapeutic strategy, aimed at combating chronic inflammation and tissue damage with aging. The findings pave the way for novel rationalized intervention strategies against age-related chronic inflammatory disorders.
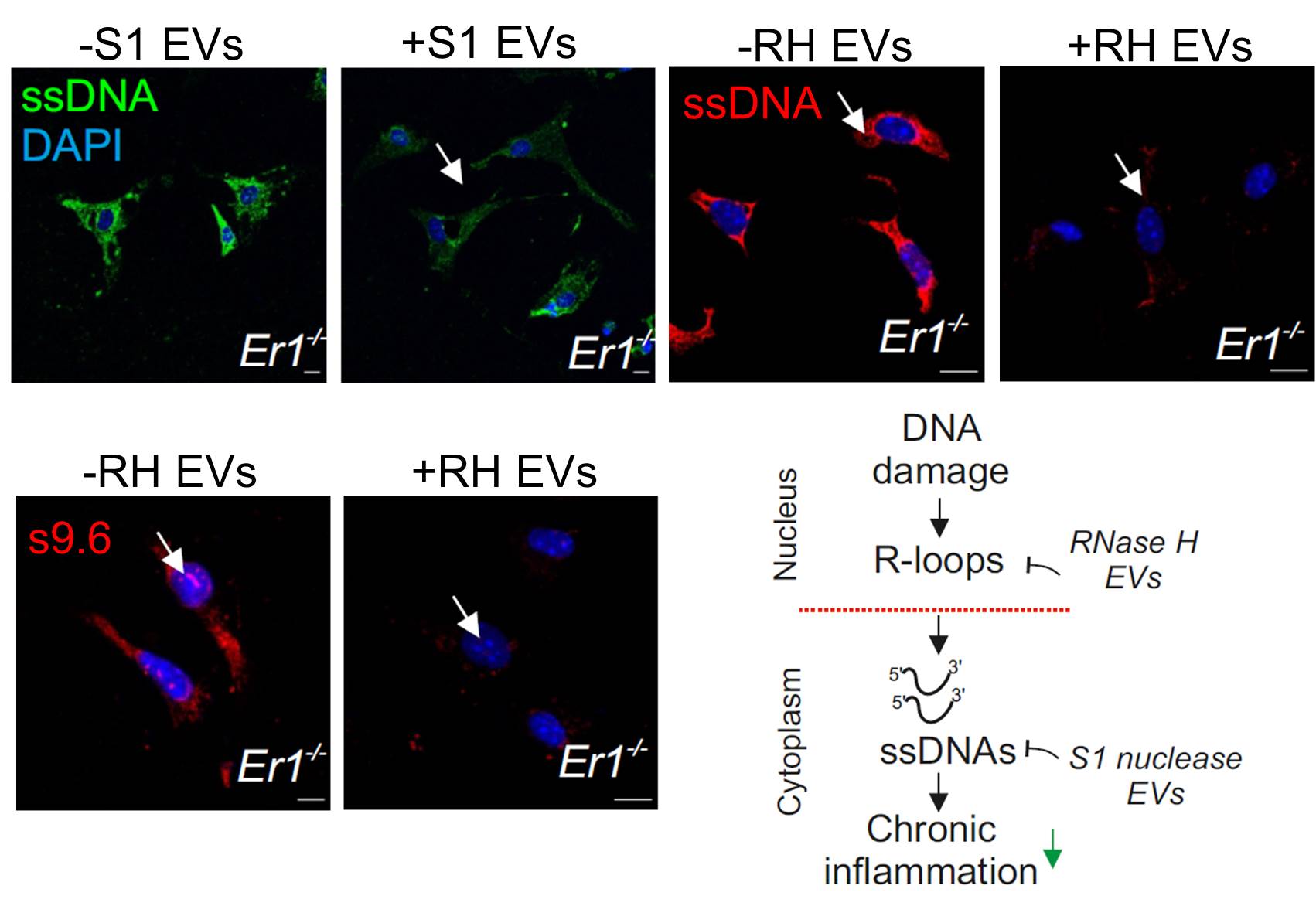
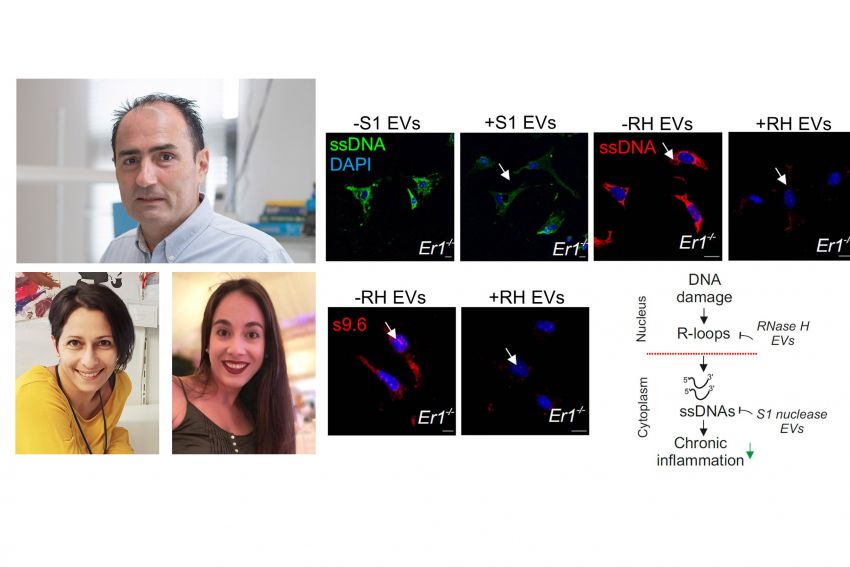
An extracellular vesicle (EV)-based strategy to deliver recombinant S1 or ribonuclease H nucleases in inflamed Ercc1−/− pancreatic cells.
Treatment of Ercc1−/− animals with the EV-delivered nuclease cargo eliminates DNA damage-induced R-loops and cytoplasmic ssDNAs alleviating chronic inflammation.
Using ERCC1-defective animal models of the human progeroid syndrome XFE, the IMBB researchers revealed that the gradual accumulation of irreparable DNA lesions leads to the premature onset of chronic pancreatitis in the DNA repair-deficient animals. Further work in cells from these animals revealed that DNA damage triggers the formation of RNA: DNA hybrids called “R-loops” causally contributed to the release and build-up of single-stranded DNA fragments in the cytoplasm of cells. In turn, the cytoplasmic DNA fragments stimulated a viral-like immune response in the pancreas of DNA repair-defective and naturally aged mice. To reduce the proinflammatory load, the IMBB researchers developed an extracellular vesicle (EV)-based strategy to deliver recombinant RNase H or S1 nuclease in inflamed Ercc1-/- pancreatic cells in vitro and in vivo. Using this novel strategy, they found that treatment with the EV- delivered nuclease cargo rapidly removes R-loops and the ssDNA moieties in the cytoplasm of pancreatic cells, thereby reducing the proinflammatory response seen in the DNA repair deficient mice.
The findings support the notion that the development of EV-based therapeutic regimens against DNA damage-driven cytoplasmic ssDNAs is a promising therapeutic strategy against chronic inflammation and tissue degeneration with aging.
The research was recently published in Science Advances: https://www.science.org/doi/10.1126/sciadv.abj5769
For more information, please contact:
- George A. Garinis, Professor, Dept. of Biology, University of Crete and affiliated group leader at FORTH- IMBB, http://www.garinislab.gr, tel.: +30 2810391246, email: garinis@imbb.forth.gr
- Kalliopi Stratigi, postdoctoral researcher FORTH-IMBB, tel.: +30 2810 391072, email: callina@imbb.forth.gr